South Australian Medical Heritage Society Inc
Website for the Virtual Museum
Home
Coming meetings
Past meetings
About the Society
Main Galleries
Medicine
Surgery
Anaesthesia
X-rays
Hospitals,other organisations
Individuals of note
Small Galleries
Ethnic medicine
- Aboriginal
- Chinese
- Mediterran
Radiotherapy and Bragg’s PeakHow application of Bragg’s finding in 1904 is changing the practice of radiotherapy today
Acknowledgment: We are grateful to Associate Professor Eva Bezak, chief physicist in the Department of Medical Physics at the Royal Adelaide Hospital (RAH) for the suggestion for this web page and also for relevant information.
The discovery of X-rays by Conrad Wilhelm Roentgen in 1895 revolutionised not only the diagnostic but also the therapeutic fields of medicine. Clinicians soon noticed that exposure to X-rays decreased the size of tumours. Thus skin and breast tumours were treated as early as 1898.


Also at this time Becquerel (1895) discovered natural radioactivity and Marie Curie isolated radium (1898) and clinicians started to use it for local treatment of tumours. Dr. Donald Beard records that one of Sir Henry Simpson-Newland’s first task on his appointment to the staff as Honorary Radiographer in 1908, was to supervise the distribution and application of Radium.
Apart from improving the safety, power and directing of the X-ray beam not much has changed until the end of the second world war when nuclear reactors started producing artificial radio-elements. The first element used in radiotherapy was Cobalt 60 in 1951 which produced gamma rays. Another source of more energetic X-rays was the linear accelerator (1952) in which the electrons were accelerated by the changing electric fields of radio frequencies. Both produced better results due to the better penetration by the higher energy of the photons, (X-rays). Precise localising of the beam to the tumour was not easy unless the lesion was superficial and localised.
The development of computers produced further advances. Hounsfield developed the Computer assisted Tomography (CT) Bloch and Purcell discovered nuclear magnetic resonance which led to the development of MRI (1970s). Nutt and Townsend were responsible for the development of the Positron Emission Tomography (PET) (1990s)
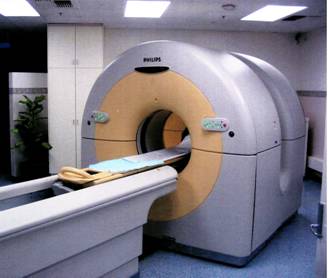
A PET scanner installation in the Nuclear Medicine Department of the Royal Adelaide Hospital
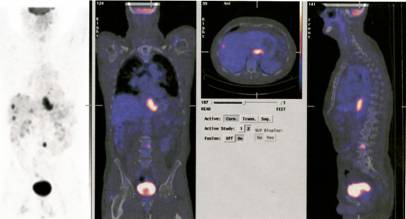
Now one can use CT and PET scan combinations to improve the localisation and identification of deep-seated tumours. An additional bonus due to the use of computers is that the beams can be directed on the centre of the lesion during respiratory movement. The most recent advance is based on Bragg’s discovery some 100 years ago. In the last ten years radiotherapists started to use protons neutrons and other atomic nuclei. Such as Tritium and carbon. These particles release their energy at predetermined depths “Braggs Peak” by the predetermined energy of the particles and thus prevent damage to the adjacent tissues.
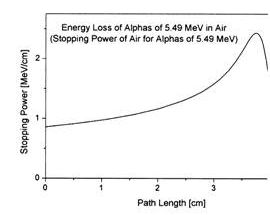
William Bragg published curves for ionization of air by radium alpha particles (1904) demonstrating that the ionization peaked at an increased depth , an unexpected finding.

William Bragg was the professor of physics and mathematics in Adelaide University 1896 -1909
The phenomenon is exploited in particle therapy of cancer, to concentrate the effect of light ion beams on the tumour being treated while minimizing the effect on the surrounding healthy tissue. The blue curve in the figure ("modified proton beam") shows how the original mono-energetic proton beam with the sharp peak is widened by increasing the range of energies, so that a larger range of depths can be treated. This is typically achieved by using variable thickness wedge attenuators.
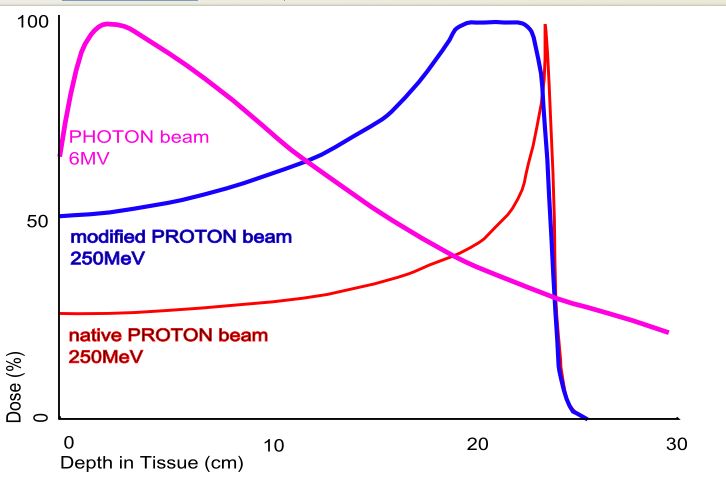
Proton beam therapy delivers a high dose of radiation to a very localized site. Protons, being mass particles unlike x-rays, slow down faster than photons. They deposit more energy as they slow down, culminating in a peak (the Bragg peak). This allows the majority of radiation to be delivered to the target site with less scattering of radiation around and beyond to the adjacent normal issues.
Proton beams can be conformed [shaped in three dimensions] to fit the target area. The beam can be carefully shaped to the dimensions of the tumour, and so deliver most of the radiation to the targeted tumour mass, not to the surrounding normal tissue.
Proton beam radiotherapy contrasts with conventional X-ray or Gamma ray radiation therapy [often called PHOTON beam] due to the unique properties of minimal scatter as the proton beams pass through the tissue, and deposit the ionizing energy at a precise depth (the Bragg peak). Thus radiation exposure to surrounding normal tissues is minimized, permitting higher radiation doses to the target area and improved local control, with less damage and side effects.
Protons, which are positively charged subatomic particles, deposit energy differently than x-ray beams do. Compared to an x-ray beam, a proton beam that is delivered with sufficient energies (or "modulated") has a low "entrance dose" (the dose in front of the tumour), a high-dose "Bragg peak" region, which is designed to cover the entire tumour, and no "exit dose" beyond the tumour. In contrast, X-ray beams may deposit most of their dose in tissues in front of the tumour, and continue to penetrate through the body after passing through the target area.
Accelerating protons (with a large mass nearly 2,000 times that of an electron), requires heavy equipment - weighing into the hundreds of tons. For instance, the Orsay proton therapy center, in France, uses a synchrocyclotron to accelerate the protons. It weighs 900 tons in total. It was built for particle physics research and later converted for medical use.
Control room of the Orsay proton accelerator
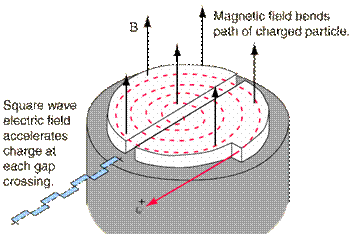
A cyclotron is a machine used to accelerate charged particles to high energies. The first cyclotron was built by Ernest Orlando Lawrence and his graduate student, M. Stanley Livingston, at the University of California, Berkley, in the early 1930's.
A cyclotron consists of two D-shaped cavities sandwiched between two electromagnets. A radioactive source is placed in the center of the cyclotron and the electromagnets are turned on. The radioactive source emits charged particles. A magnetic field can bend the path of a charged particle so, if everything is just right, the charged particle will circle around inside the D-shaped cavities. However, this doesn't accelerate the particle. In order to do that, the two D-shaped cavities have to be hooked up to a radio wave generator. This generator gives one cavity a positive charge and the other cavity a negative charge. After a moment, the radio wave generator switches the charges on the cavities. The charges keep switching back and forth as long as the radio wave generator is on. It is this switching of charges that accelerates the particle.
A proton has a positive charge so its path will be bent by magnetic fields. As a proton goes around the cyclotron, it crosses the gap between the two D-shaped cavities. If the charge on the cavity in front of the proton is negative and the charge on the cavity in back of it is positive, the alpha particle is pulled forward (opposite charges attract while like charges repel). This accelerates the proton. The proton travels through one cavity and again comes to the gap when the radio wave generator has changed the charges on the cavities. The proton once again has a negative charge in front of it and a positive charge behind and is again pulled forward. This is how a cyclotron accelerates particles! The faster a charged particle moves, the less it is affected by a magnetic field. So, as particles speed up in a cyclotron, they spiral outwards. This makes it easy to get the particles out of the cyclotron, but also puts a limit on the amount of acceleration.
A modern Cyclotron for radiation therapy (from Wikipedia, http://en.wikipedia.org/ /Image:Cyclotron.jpg)
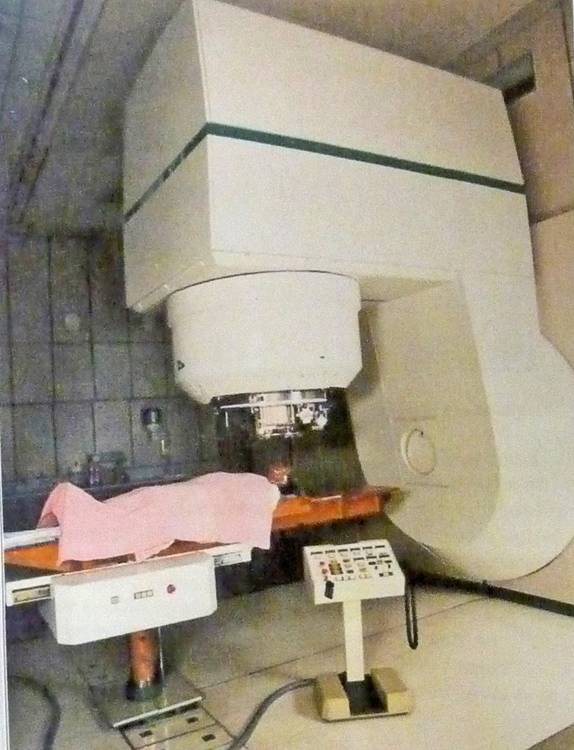
Gantry & patient of the proton therapy machine as used by Medical Radiation Group at the Groote Schuur Hospital in Capetown
Clinical reports
The first reports of clinical studies assessing the value of proton radiotherapy were reported in the 1980s. The reports were mainly from Japan and involved the treatment of non small cell lung cancers “NSCLC”, liver tumours, prostate cancer and brain and spinal chord tumours. The reports were optimistic particularly regarding neural tumours, but usually ended with the phase More studies are needed.
These were the early stages of the learning curve. The reports published this century are enthusiastic particularly as regards chordomas and brain lesions due to absence of damage to adjacent structures. Good results are reported of the treatment of ”NSCLC”, prostatic and salivary gland tumours. There is no doubt that the decreased energy loss beyond the Bragg Peak reduces tissue damage to the adjacent tissues.
Most recently several centre's commenced using atomic nuclei heavier than hydrogen ie. Carbon. During his recent visit to Adelaide Professor Oliver Jakel presented his good results using carbon nuclei in the treatment of spinal chord and brain tumours. Unfortunately USA & UK do not finance this kind of therapy. This therapy is still confined to centres in Germany, Japan, Austria and France. The potential appears to be exciting but More studies are needed.
-o0o-