South Australian Medical Heritage Society Inc
Website for the Virtual Museum
Home
Coming meetings
Past meetings
About the Society
Main Galleries
Medicine
Surgery
Anaesthesia
X-rays
Hospitals,other organisations
Individuals of note
Small Galleries
Ethnic medicine
- Aboriginal
- Chinese
- Mediterran
History Of The Measurement Of Blood Pressure
The Role Of Mercury 200Hg
ACKNOWLEDGEMENT
We are grateful to our physician, Dr. Hughes, and to Prof. Donald Simpson for allowing us to photograph their sphygmomanometers.
Pressure Measurement
Advances in anatomy, histology and physiology were directly related to the technology of the day. Thus relaxation of religious constraints helped the progress of anatomy and macroscopic pathology.
The increase of knowledge in pathology and physiology came only with the advent of microscopy and advances in physics. The measurement of blood pressure is directly related to Evangelista Torricelli, who was the first to measure atmospheric pressure.
The barometer (from the Greek baros "weight", and metron via the French mètre, "meter") is generally considered to have been created by the Itallian scientist and mathematician Evangelista Torricelli around 1643. He was trying to discover why vacuum pumps could not raise water more than 10 metres. Knowing that mercury was approximately 13 times denser than water, he was able to scale the experiment down to a more manageable size. He filled an approximately 1 metre long tube, sealed at one end, with mercury, and then placed it open side down in a bowl or mercury. The height of the mercury in the tube then dropped to about 760mm above the mercury in the bowl, and varied over time, and was later found to depend on altitude. This lead to the idea of air having mass, and hence of atmospheric pressure.
The force of the mercury in the column must be balanced by the force of the mercury in the bowl pushing into the column, which depends on the atmospheric pressure. The atmospheric pressure could then be determined from two simple equations: F=ma (force = mass x acceleration) and F=PA (force = pressure x area).
Fair = FHg
PairAair = mHga
substituting mass = volume * density, and volume of a cylinder = cross-sectional area * height, and g = acceleration due to gravity, and ρ is the density of mercury:
PairAair = AHghρa
Since we are balancing the forces inside the tube, Aair = AHg = A = cross-section area of the tube.
PairA = Ahρg
Pair = ρgh
The density of mercury is 13,545 kg/m3 at 20°C (or 13,595 at 0°C), so we can now calculate that the air pressure was approximately 101 kPa.
Because the weight of the mercury column is dependant on both the density of mercury (which in turn depends on the temperature), and the local gravity (which varies with altitude, etc), the barometer needs to be calibrated for maximum accuracy if is not used at sea level and near room temperature.
The low vapour pressure (essentially, the volatility) of mercury allowed for a good vacuum to form, and its low viscosity and surface tension were also beneficial, especially in the small tubes of later meters. As mercury has a very linear relationship between temperature and density (eg: see mercury's physical properties at The Engineering Toolbox), it was also well suited to use in thermometers.
In recognition of Torricelli's work, the pressure in a vacuum is measured in torr (1 Torr = 1/760 standard atmospheres, and extremely close to 1 mmHg). The pressure above the mercury column in Torricelli's experiment is known as a Torricellian vacuum, and is approximately 10-3 Torr (0.13 Pa), comprised of mercury vapour. In 1959, the USSR honoured Torricelli by placing his image on a stamp.

"CCCP Post, 40 kopeks.
Outstanding Italian physicist and mathematician. Evangelista Torricelli"
Source: Wikipedia
A later improvement to the barometer came in 1844 from the French scientist Lucien Vidi. He created the aneroid barometer (meaning "without fluid"), which uses a flexible sealed metal box, called an aneroid cell. Often several of these cells are stacked together to increase the amount of movement. These cells are then linked via a series of levers, further increasing the movement, to a dial displaying the pressure.
-
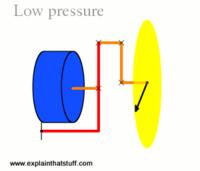 Low pressure.
Low pressure.
Source: Explain That Stuff -
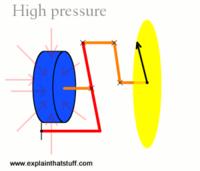 High pressure.
High pressure.
Source: Explain That Stuff

Source: Wikipedia
Another common type of aneroid sensor was patented in 1849 by Eugene Bourdon, and became known as a Bourdon gauge. He realised that a curved tube will try to straighten when it is pressurised. One end of the tube is attached to the input, and immobilised. The other end is free to move, and is linked to the display by a series of levers and gears, magnifying the distortion.
Blood Pressure Measurement
-
 A USSR stamp of William Harvey from 1957
A USSR stamp of William Harvey from 1957
"The Outstanding English physician who discovered blood circulation.
William Harvey"
Source: Wikipedia
The estimation of blood pressure was due to the discovery of blood circulation by William Harvey in 1628. He published a treatise entitled "Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus" ("An Anatomical Exercise on the Motion of the Heart and Blood in Living Beings") in which described the heart as a pump, and arteries as efferent and veins as afferent pipes (vessels).

One of the first to measure blood pressure was Stephen Hales in 1727. Among his other achievements (eg: measuring the volume of the heart), Reverent Hales is noted for siting a cannula in the leg artery of a 14 year old mare, connecting it to a long glass tube and measuring the height to which the blood rose. After some time the blood rose to 8 feet and 3 inches above the ventricles, and oscillated 3-4 inches with each contraction (Hales, S. "Haemastaticks", 3rd Edition, 1738).
-
 Vierordt's sphygmograph.
Vierordt's sphygmograph.
Source: Wellcome Library.
In 1855, Karl von Vierordt asserted that blood pressure could be measured by non-invasive means by compressing the arteries with weights, and measure the pressure required to stop the blood from flowing.
Vierordt's design was difficult to use, and largely unsuccessful. It was significantly improved by Étienne Jules Marey in 1860. Marey by replacing the counterweight with a water filled chamber, in which the pressure could be increased, and used a sphygmograph to record the pulse, and a manometer to record the blood pressure. Marey's design improved the accuracy of measurement and recording, but was still very complicated to operate, and wasn't used often.
-
 Marey's sphygmograph.
Marey's sphygmograph.
Source: Wellcome Library. -
 A diagram of Marey's sphygmograph.
A diagram of Marey's sphygmograph.
Source: Wellcome Library.
The first recorded direct measurement of human blood pressure was made by the French surgeon Faivre in 1856. He connected the brachial and femoral arteries of a patient to a mercury manometer, and recorded their pressures.
The first modern sphygmomanometer (from the Greek "sphygmos", pulse; and French "manomètre", pressure meter) was developed in 1881 by the Austrian physician Samuel Siegfried Karl Ritter von Basch. Basch was a graduate of the Karl University in Prague and the physician to Maximillian, the Emperor of Mexico. Basch used a water filled bag surrounding a rubber bulb which was connected to a column of mercury. This was placed on an artery in the upper arm. Water was pumped into the bag until the pulsation stopped. The height of the mercury column in millimetres was then recorded as the systolic blood pressure. Von Basch's device was experimentally confirmed to be more accurate than earlier devices.
In 1889, Pierre-Carl Potain improved on Basch's design, by replacing the water with air, and using an aneroid meter instead of a mercury manometer.
-
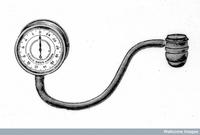 Basch's sphygmomanometer.
Basch's sphygmomanometer.
Source: Wellcome Library. -
 A drawing of Basch's sphygmomanometer in use.
A drawing of Basch's sphygmomanometer in use.
Source: Wellcome Library.
-
 Scipione Riva-Rocci.
Scipione Riva-Rocci.
Source: Wikipedia. -
 A diagram of Riva-Rocci's cuff in action.
A diagram of Riva-Rocci's cuff in action.
Source: Wellcome Library.
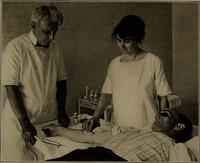
The Basch design was later improved by Scipione Riva-Rocci in 1896, who made it easier to use. He created a non-expanding cuff that encircled the whole arm, and had an inflatable bag on the inside. This bag was then connected to both a rubber bulb, which inflated it, and to a mercury manometer to measure its pressure. The patient's arm was placed through this ring, and then the bag was inflated until the pulse stopped. The pressure was slowly decreased until the pulse could be felt again, which was then recorded as the systolic pressure.
In a report to the Imperial Military Medical Academy in 1905, the Russian surgeon Nikolai Korotkov described a non-invasive way to measure blood pressure. He wrote that by inflating a cuff wrapped around the upper arm until circulation was stopped, and then slowly reducing the pressure, one could measure both the systolic and diastolic blood pressure. A stethoscope placed under the cuff was used to listen to the blood flow.
As normal blood flow is non-turbulent, it does not make any sound. The sounds that can heard are caused by the turbulent flow of blood as it passes through the intermittently opened artery.
The first tapping sounds occur when the cuff pressure is the same as the systolic (maximum) blood pressure, and the blood is able to pass through. Eventually, as the pressure in the cuff is decreased, there are no more sounds produced. This indicates that the blood is flowing freely, which gives the diastolic (minimum) blood pressure.
These sounds have become known as Korotkov sounds and are still widely used.
After the arrival of the microprocessor, it has been possible to measure the pulse digitally. These machines often use a piezoelectric pressure sensor to measure the oscillations caused by the pulse, and then calculate the systolic and diastolic pressures. This enables them to be used in areas too noisy to use the analogue auscultation method, and also removes the need for the hazardous mercury. Unfortunately, they may not be as accurate (depending on the size of the cuff, placement, etc), and there are a number of cases where the use of these digital machines is inadvisable, such as arteriosclerosis, arrhythmia, pulsus paradoxus, etc; and in these cases a trained operator using an analogue sphygmomanometer is more accurate.
For a more detailed history, you may wish to read "A Short History of Blood Pressure Measurement" by Jeremy Booth, in the "Proceedings of the Royal Society of Medicine", vol 70, Nov 1977.
Blood Pressure Meters
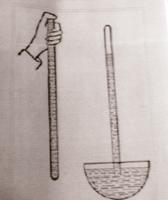
A mercury manometer kindly loaned to us by professor Donald Simpson. It belonged to his aunt, Dr. Hubbe. She studied arts and medicine at the University of Adelaide, taught anatomy in Professor Watson's department, and had a small private practice.
The gauge measures to over 220 millimetres of mercury. The rubber bulb is used to inflate the cuff and the meter is contained in the stainless steel container (the dial). The knurled screw on the inflating bulb is used to control the flow of air into and out of the meter.
Three blood pressure cuffs. Those attached to the aneroid and mercury manometers are connected to two tubes. One tube connects to the inflating rubber bulb and the other to the manometer. The top centre one has only one tube, and is connected to a digital manometer.
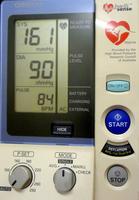
Above is a digital manometer purchased from the local chemist for 90 dollars. The cuff is inflated by a battery operated pump, and the systolic and diastolic pressures are recorded digitally during the deflation and stored. They reported as reliable and accurate, but some physicians still use mercury or aneroid types.
A professional blood pressure meter, which comes with a range of cuff widths for more accurate readings over a wider range of arm diameters, allowing for more accurate readings.
Some modern blood pressure monitors are integrated with other devices, and are able to record pulse, blood pressure, oxygen saturation, temperature and an ECG. These data can be printed or stored for later analysis and reference.
Addendum: Mercury
Mercury has been known and used since ancient times, with evidence of it in Egyptian tombs dating to around 1,500 BCE, and its ores used for decoration from at least 8000-7000 BCE. It has been used for many and diverse purposes, perhaps the best know use is as the working fluid in thermometers.
Decorative uses
Mercury(II) sulphide, better known as cinnabar, was used to create the red pigment vermillion, which was used in cosmetics in classical Rome and Egypt, to decorate ceramics, in illuminated manuscripts and in other paintings and decorations.
Cinnabar was also used for its colour in funerals. For example, the Mayans considered red to be the colour of death and rebirth, and often covered their dead (eg: the tomb of the Red Queen) with powdered cinnabar. There have been traces of vermilion found in burials dating to around 8000-7000 BCE at the neolithic village of Çatalhöyük in what is now Turkey.
Metallic mercury was used in fire gilding. Small grains of gold were heated, and added to hot mercury, and then stirred with an iron rod. This amalgam was then cooled, and the excess mercury was squeezed out. The resulting product could then be painted or smeared onto an object. The amalgam was then carefully heated to evaporate the mercury as a toxic gas, and slightly melt the gold. As one would imagine, this caused several health problems, and was eventually replaced by electroplating. The object then underwent further processing to bring out gold's luster.
Scientific uses
Mercury also has several scientific uses too, the most familiar being as the working fluid in thermometers and barometers. The triple point of mercury (-38.83440 °C and at 0.2 mPa) can also be used to calibrate thermometers.
Mercury in the form of mercury(II) oxide was used by both Carl Wilhem Scheele (1771-2) and Joseph Priestly (1774) in their experiments. By heating the HgO in a vessel, they liberated a gas, which Priestly called "dephlogisticated air". It was noted that this gas made candles burn brighter, and mice could survive longer when breathing this gas compared to normal air. This gas was later called "oxygène" by the French Antoine Lavoisier in 1777.
The noted English scientist Michael Faraday used a pool of mercury in the first electric motor in 1821.
Spinning a container of liquid mercury at a constant speed forms a parabolic reflective surface, which can be used as the primary mirror in a liquid mirror telescope. It does have the ever so slight disadvantage of only pointing upwards.
Mercury can also be used as a reference electrode.
Industrial uses
There have been several industrial uses for mercury. These include the production of chlorine and sodium hydroxide (the chloralkali process, or Castner Keller process); gold and silver mining (mercury forms an amalgam with the metals, allowing for easier collection and processing of small particles), in the production of felt, in mercury cell batteries, in detonators (mercury fulminate), as a antifungal agent, in anti-fouling paints, and in mercury switches, to name a few.
Mercury vapour is also used in fluorescent lights, much the same as the more famous neon lights. One of the early forms was the Geissler tube. When subjected to a large voltage difference, mercury vapour emits short-wave ultraviolet light. This can be used as is, as in the case of UV and germicidal lamps; or the inside of the tube can be coated with a mixture of phosphors, which are excited by the UV light to emit visible light (eg: compact fluorescent lights).
Medical uses
Mercury has had several medicinal uses, of varying effectiveness. One prominent use was in forming amalgams with gold, and used in dentistry to fill cavities, but its use has been severely limited, if not outright banned in recent years. It has also been used in small quantities as a preservative (eg: Thiomersal/Thimerosal) in vaccines. Other uses included as an ingredient in some laxatives, diaper-rash ointments, topical antiseptics (eg: Merbromin, sold as Mercurochrome and Merbromine), and as biological tissue dye.
Source: Wikimedia.
Other less useful, if not outright harmful applications ranged from immortality pills (which are credited in the death of the first Chinese emperor Qin Shi Huang, whose tomb, according to legend, has 100 rivers of mercury), as a fertility treatment, and for treating skin ailments. Mercury was (in)famously used treat syphilis (eg: mercury(I) chloride (aka calomel), and blue mass (which was also used in many other conditions too)), from the 16th century, up until the early 20th when antisyphilitics became available.
Source: Wikimedia.
Calomel was also used in teething powders until the mid 1950's. This resulted in many cases of mercury poisoning. Symptoms included itching, rashes, and swelling, and became known as pink disease, or acrodynia.
Mercury poisoning
The toxic nature of mercury has been known since antiquity, with slaves and convicts being sent to the Roman cinnabar mines known to have greatly reduced life expectancies. The German chemist Alfred Stock performed much research on mercury and mercury poisoning around the 1920's and 30's, and tried to stop the use of mercury and its derivatives. Through his work, Stock was exposed to low levels of mercury vapour over a long time, and endured chronic mild poisoning. He described symptoms of poor memory, lethargy, and depression.

Source: Wikimedia.
Perhaps the best know instance of mercury poisoning occurred in Minamata city, Kumamoto prefecture, Japan, and discovered around 1956. Methylmercury contaminated wastewater was dumped into the sea from 1932 to 1960. This bioaccumulated in the seafood, some of which was eventually caught and eaten, causing mercury poisoning, and became known as "dancing cat fever", or Minamata disease, officially claiming 1,784 lives. Symptoms included rashes, ataxia, numbness, general muscle weakness, peripheral blindness, paralysis, insanity, and could lead to death.
Treatment
Some mercury can be naturally expelled after a time, as in the case of mercury diuretics and laxatives. There are some compounds that can be used in chelation therapies. These include dimercaptosuccinic acid (DMSA), 2,3-dimercapto-1-propanesulfonic acid (DMPS), D-penicillamine (DPCN), and dimercaprol (BAL).
As you can see, several of these are mercaptan-based compounds. Mercaptans are organosulphur compounds a with carbon-bonded sulphydryl (-SH) group, and are also known as thiols. They derive their name from the Latin mercurium captāns, meaning "capturing mercury", for their ability to strongly bond onto mercury compounds. They are also used as an odorant to make natural gas noticeable (the rotten egg smell).
Unfortunately, the neurological symptoms of severe poisoning seem to be irreversible.
Conclusion
Mercury has been used for several thousands of years for many reasons, and its toxic nature has been known since at least classical times. Thanks to scientific advances, mercury has largely been replaced by safer compounds in many of these uses, but it still retains some niche uses (eg: in high accuracy thermometers and barometers).